Develop the Clinical Trial Protocols of the Future Using AI
Protocol AI TM software is an end-to-end solution that enables experts to design and develop high-quality protocols and study plans rapidly and efficiently, helping companies shorten clinical development timelines and accelerate the delivery of new treatments to market.








Reduce Time to Market with Protocol AI TM
Accelerate market access with optimized trial design and faster protocol and study plan creation and implementation. We are setting new standards for precision and excellence in the industry.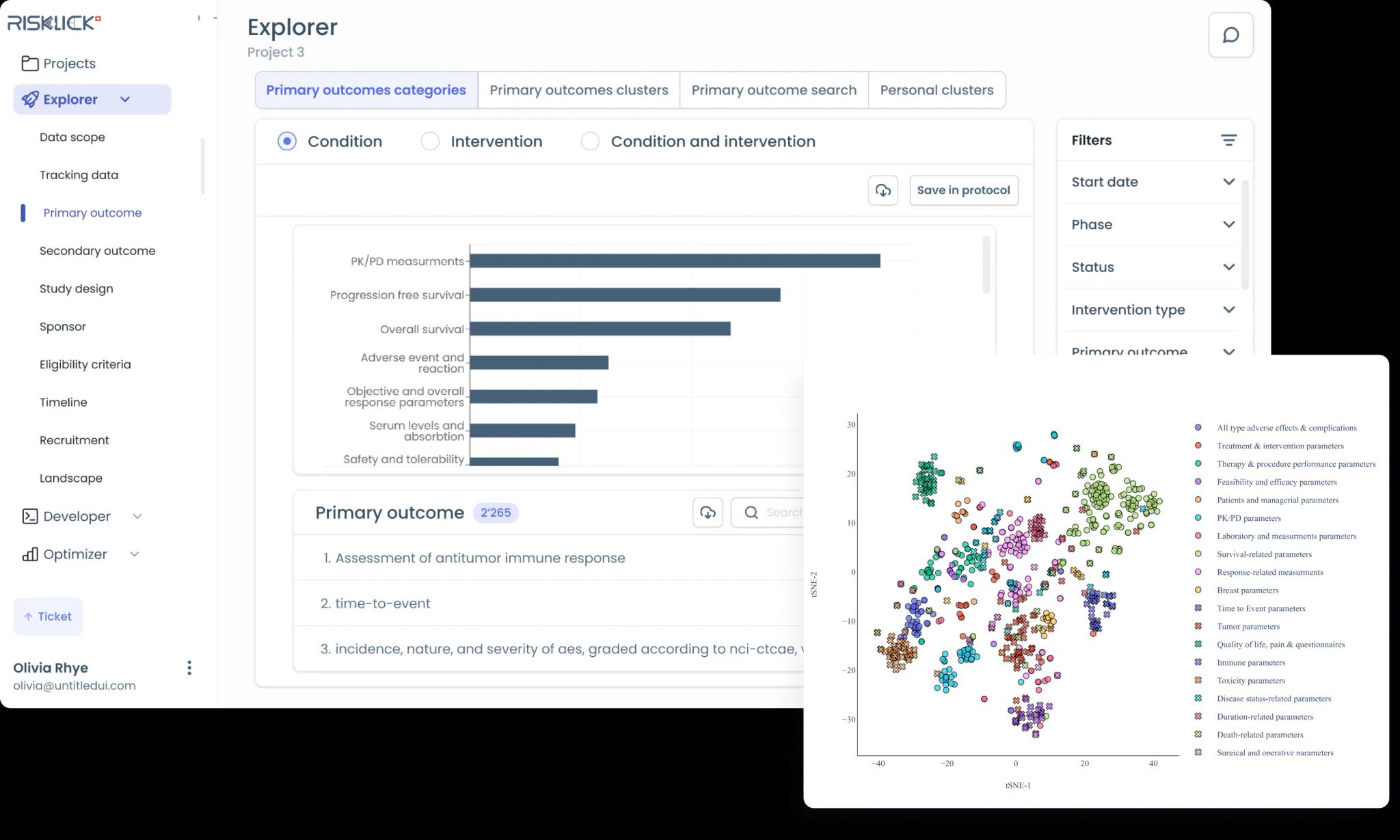

Sven is affiliated with the University of Bern and was director of CTU Bern, the clinical trials unit of the University of Bern, for several years. He has extensive expertise in the design and conduct of innovative clinical trials, especially randomized-controlled trials.

Beat was the Initiator and former leader of the Roche Pharma Clinical Quality Risk Management (C-QRM) project for clinical development and pharmacovigilance for 25 years. Beat led more than 150 clinical trial center audits worldwide.

Matthias Egger is an internationally renowned epidemiologist with vast experience in clinical trial methodology, meta-analysis, and evidence generation. He is a professor of epidemiology at the University of Bern and the University of Bristol. Additionally, he serves as the President of the National Research Council at the Swiss National Science Foundation.

Ingrid is a physician specialized in General Medicine, Clinical Pharmacology, and Pharmaceutical Medicine, with over 30 years of experience in various senior medical, operational, and managerial roles within the pharmaceutical industry, CROs, and clinical trial sites. Her focus revolves around clinical trial design and management, along with ethical and regulatory aspects.




